Pet FAQs
Click on the question below to see the answer..
LubriSynHA is a premium, liquid oral joint supplement composed of high-molecular weight hyaluronic acid. Hyaluronic acid is essential to healthy joints because it works to support healthy synovial fluid.
Hyaluronic acid (HA), also known as hyaluronan, is a clear, viscous substance that is naturally produced by the body of all mammals. The largest amounts of it are found in skin, eyes, and joints. Its main function is to retain moisture to keep tissues well lubricated.
It is the main component of synovial fluid, which works to cushion healthy joints. With age and injury, this fluid starts to break down causing pain and inflammation. Consuming a high-molecular weight, liquid hyaluronic acid daily can work to replenish this fluid and greatly reduce discomfort.
While glucosamine and chondroitin have been known to help keep connective tissues (cartilage and ligaments) healthy, it does not support the synovial fluid. HA is the only ingredient that does this.
High-molecular weight hyaluronic acid is proven to be more bio-identical to what the body naturally produces. Therefore, it is more readily absorbed by body and distributed to parts of the body that need it (i.e. inflamed joints).
LubriSynHA has a molecular weight of 2.4 million Daltons. We pride ourselves on having the highest molecular weight and quality HA on the market today.
LubriSynHA is in a liquid form for a few reasons:
- Hyaluronic acid in its natural state is a liquid. When it is put in a pill or powder, it is dehydrated. When consumed, the body has to re-hydrate in order for it to be used. Most of the time, it is passed to the lower GI before this happens. Therefore, results are slower and sometimes not as effective.
- Liquid HA is far more easily absorbed by the body naturally. LubriSynHA is absorbed in the upper GI (mouth and upper mucosal) and distributed to the body. Because of this, results are often seen much more quickly than pills or powders.
LubriSynHA is safer and less invasive than injectable products and can show much more consistent results. It is more effective than most supplements on the market because of the quality of our HA as well as supporting the most integral part of the joint, the synovial fluid.
Because of the way they quickly metabolize things, the dosage for pets varies by their weight. The dosage can be viewed below. We do recommend doubling the dosage for the first week to ensure it appropriately gets into their systems.
Simply put on top of their food or administer with a syringe into their mouth.
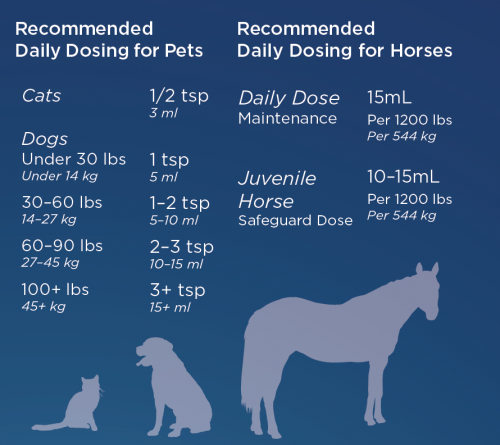
Because it has a slightly sweet taste from the glycerin, a natural sweetener, we rarely have animals that don’t like the taste. You can top-dress it on their food or give it by oral syringe.
There is 75mg of high-molecular weight hyaluronic acid in a daily dose of LubriSynHA.
At MSRP, LubriSynHA costs around $1.10 per day when dosed as recommended. You can often find it for less than that.
Unlike other forms of liquid HA made from animal sources (e.g. rooster combs), LubriSynHA is extracted from a microbial source in an FDA approved facility. This method not only keeps LubriSynHA free from protein contamination that can cause HA products to break down and lose their effectiveness, but also ensures it is a vegan product.
Anytime! LubriSynHA works as a preventative on younger pets, working to maintain healthy joint function and also on older pets currently suffering from joint discomfort.
Because of our high-quality HA and liquid formulation, LubriSynHA works quickly and most people see results in as little as 7-10 days.
All LubriSynHA products have a shelf life of 18-months.
Yes, because it is all-natural it is perfectly safe to administer LubriSynHA in conjunction with any other supplement or medications without interactions.
Studies of high molecular weight hyaluronic acid show that it begins to appear in tissues outside the intestinal tract in thirty (30) minutes, and peaks between four and six hours post ingestion. As a daily supplement, LubriSynHA provides a continual supply of hyaluronic acid to support the rapid turnover of synovial fluid.
The main ingredient of LubriSynHA is hyaluronic acid. Our LubriSynHA + formula is fortified with MSM (Methyl Sulfonyl Methane). The inactive ingredients are water, glycerin, citric acid, xanthan gum and potassium sorbate.
LubriSynHA contains our trusted hyaluronic acid formula. The LubriSynHA Plus MSM contains the extra support of MSM which aids in the support of connective tissues and added anti-inflammatory.
MSM or Methylsulfonylmethane has anti-inflammatory and antioxidant effects. Sulphur, which is a major component of MSM, plays an important role in making collagen and glucosamine, both of which are vital for healthy joints. Our MSM product is great for older animals with more severe cases of arthritis or performance animals for accelerated recovery.
The pet & equine, and livestock formulas are the same, only dosed differently. The livestock is labeled separately as well. The human product is 20% stronger than the other formulas but contains all the same safe ingredients.
No, since it is all-natural, you cannot overdose your animal on LubriSynHA. In fact, some animals with more severe arthritis do better on a higher dose of LubriSynHA.
LubriSynHA products are available direct to end-users in the USA.
We distribute to Canadian customers through Noble Distributors Canada and Greenhawk Equestrian.
We are also available for purchase internationally as well, please contact us at customersupport@lubrisyn.com or 800-901-8498 for more information on specific countries.
If your question is not answered here, please feel free to submit it to our live chat, call our offices at 800-901-8498, or email us at customersupport@lubrisyn.com.

